What Is the Approximate Mass of One Proton
The six protons and six neutrons are stuck together. If of water is produced from the reaction of of ethane and of oxygen gas calculate the percent yield of water.

The Structure Of The Atom Boundless Chemistry
This is the approximate basic reason why iron and nickel are very common metals in planetary cores.
. One AMU also averages the masses of neutrons. What is the approximate mass of a carbon atom in atomic mass units amu. The major portion of an atoms mass nucleus consists of.
The proton mass is slightly less than the neutron mass. A4 B3 C3 D4 3An ion that consists of 7 protons 6 neutrons and 10. In imprecise terms one AMU is the average of the proton rest mass and the neutron rest mass.
Mass of Neutron in Grams. 2 Show answers Another question on Chemistry. If protons and neutrons have the same mass what is the approximate mass of one proton andor the approximate mass of a neutron in atomic mass units amu.
The mass of the proton is-Proton mass m p 16726218982110 27 Kg. The mass of a proton is 16726231 x 10²⁷ kg whereas the mass of the neutron mn 16726231 x 10²⁷ kg. Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water.
1what is the approximate mass of a proton. Other questions on the subject. Mass of a neutron 1 atomic mass unit.
Contains most of the mass of the atom. A carbon atom has a mass of about 6 amu. Neutrons are uncharged particles found within the nucleus.
That makes one AMU smaller than one free protons mass. 11 u 3 l g 2 00005 u 4 00005 g. Protons mass 1 atomic mass unit 1 u.
Chemistry 21062019 1930 haybaby312oxdjli. Mass of proton can be measured using the units kg MeVc and u AMU. All of the ice melts and the temperature of the water decreases to 00 c.
Remember to refer back to model 1. 0 0 0 5 5 a. Compared to an electron which particle has a charge.
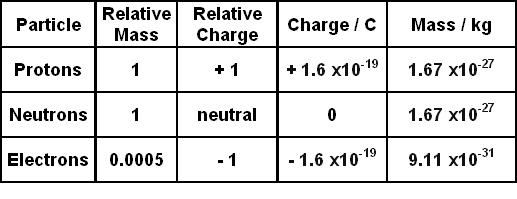
Experimental evidence indicates that the nucleus of. A proton is a stable subatomic particle symbol p H or 1 H with a positive electric charge of 1e elementary chargeIts mass is slightly less than that of a neutron and 1854 times the mass of an electron. Each electron has a negative charge -1 equal to the positive charge of a proton 1.
Aan electron and an alpha particle Ban electron and a proton Ca neutron and an alpha particle Da neutron and a proton 2Which particles have approximately the same mass. Protons and neutrons are particles located inside the nucleus of an atom and give an atom most of its mass. Therefore all we need to do is simply add 2619 to get 45 as the correct answer making choice 2 correct.
Structure of the Atom The atom is comprised of smaller particles called. The sodium atom becomes 1 a positive ion 2 a negative ion 3 an atom in an excited stale. Mass of proton is higher than the mass of.
0 0 7 2 7 a. What is the charge and mass of a proton. Mass of the proton is the sum of the mass of current quarks and the binding gluons.
How about a carbon dioxide molecule. The mass of the neutron is slightly lesser than the mass of the proton. The mass neutron 1.
0 0 8 6 5 a. What is the Approximate Mass of a Neutron. 11 u 3 l g 2 00005 u 4 00005 g Other questions on the subject.
The mass of a proton 1. A48 g piece of ice at 00 c is added to a sample of water at 74 c. The mass of electron 0.
How many grams of water were in the sample. What is mass of proton and neutron. 1 u is defined as 112 of the mass of a 12 C atombut the atomic mass of a 1 H atom which is a proton plus electron is 1007825 u so each nucleon in 12 C has lost on average about 08 of its mass in the form of binding energy.
The three subatomic particles have different charges and are. What approximate mass of on proton. One AMU is defined by having a Carbon-12 atom being 12 of them.
Answer 1 of 7. Carbon dioxide would then have a mass of 28 amu. Water molecules have a strong attraction to each other because of hydrogen bonding allowing water to move against gravity up a plants stem through capillary action.
2 An electron in a sodium atom gains enough energy to move from the second shell to the third shell. The mass of an atom is equal to the protons plus neutrons. What is the approximate mass of one proton.
The carbon-12 C-12 atom has six protons and six neutrons in its nucleus. Binding energy has been released as compared to free nucleons. A1 u B00005 u C1 g D00005 g 1What is the approximate mass of a proton.
Charge of 1 and mass of 1 amu. Protons and neutrons each with masses of approximately one atomic mass unit are jointly referred to as nucleons particles present in atomic nuclei. Chemistry 21062019 1330 kellywelly82.
Write the approximate mass of a proton neutron and electron in a. Carbon dioxide would then have a mass of 22 amu. A carbon atom has a mass of about 12 amu.
A carbon atom has a mass of about 12 amu. 14 rows 1 What is the approximate mass of a proton. Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or one Dalton.
An atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of carbon-12. Element Mass of 1 atom Average mass of 2 atoms sim Average mass of 3 atoms sim Atomic mass periodic table Beryllium Be 901218 amu 901218 amu 901218 amu 901218 amu Fluorine F 1899840 amu 1899840 amu 1899840 amu 1899840 amu.
How Does The Mass Of An Electron Compare To The Mass Of A Proton Socratic
What Is The Mass Of A Proton Quora

Gcse Chemistry 1 9 What Is The Charge And Mass Of A Proton Neutron And Electron Youtube
What Is The Relative Mass Of A Neutron Electron And A Proton Quora
Comments
Post a Comment